
Cardiovascular complications are the leading cause of morbidity and mortality in diabetic patients. As a result of the Framingham Heart Study, we now know that Diabetes Mellitus (DM) increases the incidence of Heart Failure (HF), mainly because of accelerated atherosclerosis, an increased incidence and severity of myocardial infarction, and the frequent coexistence of arterial hypertension in diabetic individuals [Kennel 1979]. However, there have also been significant numbers of cardiovascular complications occurring where typical risk factors, such as overt myocardial ischaemia and hypertension, are not present. These pathologies have been described as Diabetic Cardiomyopathy (DbCM), defined as “ventricular dysfunction in the absence of Coronary Artery Disease (CAD) or Hypertension”, and potential targeted therapies are emerging. A significant number of diabetic patients present DbCM, even though the actual incidence is difficult to estimate, since DbCM is often underdiagnosed or misdiagnosed. [Rubler 1972, de Simone 2010].
However, the advances in comprehension of molecular and cellular alterations related to DbCM, has resulted in an improved understanding of functional alterations in the diabetic heart. Data on this issue continues to reveal the significant role that mitochondrial dysfunctions play in the occurrence of this disorder.
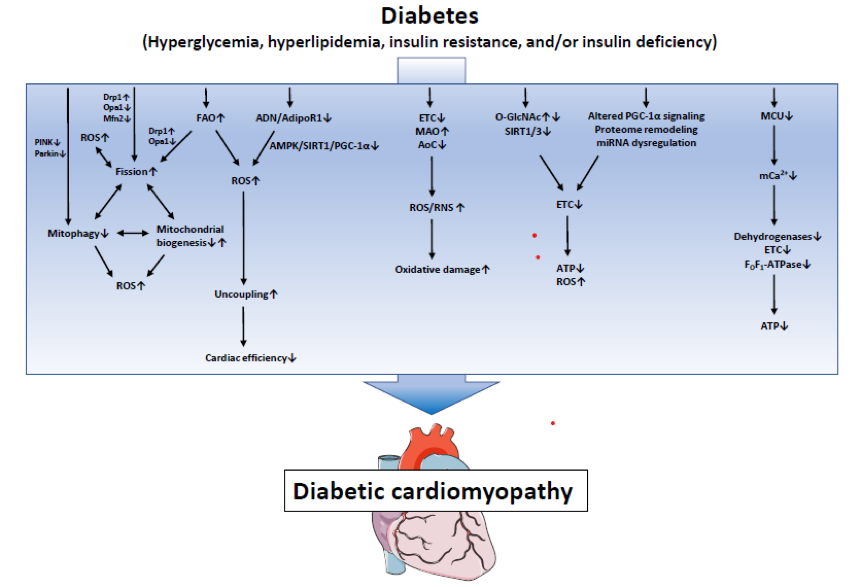
Mitochondria are the power-stations of the cell, constantly providing energy for cardiomyocytes to maintain their contractile function. Therefore it is understandable that mitochondrial defects are often observed in HF [Bugger 2014]. Alterations in mitochondrial biology have consistently been reported in DbCM, and a variety of distinct mechanisms have been proposed [Gollmer 2020].
In DM, the typically observed increase in serum Fatty Acids (FAs) and triglycerides promotes an increase in FAs uptake and oxidation. Mitochondrial uncoupling is mainly due to an excess of FAs as a substrate, causing Endoplasmic Reticulum (ER) stress and augmented Reactive Oxygen Species (ROS) production, thereby increasing oxygen consumption and diminishing ATP generation, leading to impaired cardiac contractility [Boudina 2005].
In addition to this, mitochondria are the main source of ROS in the heart, through the Electron Transport Chain (ETC). Defects in the ETC, augments monoamine oxidases activity and decreases antioxidative capacity, causing an increase of reactive oxygen/nitrogen species production and subsequent oxidative damage [Angelova 2016].
A further mechanism producing mitochondrial alteration in DbCM is the remodelling of mitochondrial proteome. The impaired expression of proteins forming ETC subunits has been proposed as a cause of impaired mitochondrial function and energy depletion in cardiac pathologies [Bugger 2010].
Post-translational modifications also contribute to mitochondrial dysfunction. Several protein targets of O-GlcNAcylation have been identified in the diabetic heart. For instance, hyperglycemia was shown to increase acylation of calmodulin-dependent protein kinase II, promoting Ca2+ release from the sarcoplasmic reticulum. Over 88 mitochondrial proteins can be O-GlcNAcylated, indicating a potential impact on various cell pathways whose impairment have been proposed to contribute to DbCM through the mis-regulation of mitochondrial biology [Ducheix 2018].
Moreover, impairment of mitochondrial homeostasis/turnover has been observed in DbCM. In animal models, a high-fat diet was shown to upregulate mitophagy, the specific targeting and removal of mitochondria by autophagy. This revealed mitophagy as a protective compensatory mechanism in DbCM by which dysfunctional mitochondria can be eliminated. Removal of mitochondria by mitophagy needs to be counterbalanced by mitochondrial biogenesis to maintain mitochondrial content of the cell. In T2DM, mitochondrial biogenesis may be reduced in different organs, including the heart [Ren 2010].
Besides, unbalance of the so-called mitochondrial process “fission and fusion”, could also lead to impaired mitochondrial homeostasis. The mechanism involves a chronic decrease in peroxisome proliferator-activated receptors (PPARs) expression in DM - normally responsible of FAs oxidation - inducing mitochondrial fission, which in turn results in mitochondrial respiratory dysfunction, increased mitochondrial ROS generation and mitochondria-dependent apoptosis [Hu 2019].
Another mechanism of mitochondrial driven cardiomyopathy in DM is the impairment of mitochondrial Ca2+ handling. Ca2+ is the key messenger in cardiomyocytes, regulating excitation-contraction coupling in the heart. Clinical and experimental data show that Ca2+ handling is impaired in DbCM, due to decreased expression of channel proteins regulating transport and reuptake of Ca2+ in mitochondria, a phenomenon accompanied by mitochondria-dependent intrinsic apoptosis [Pereira 2014].
Eventually, dysregulation of a large number of microRNAs is involved in the pathogenesis of cardiovascular diseases, including HF and DbCM. microRNAs are single strand, non-coding, small RNA molecules regulating protein levels by inhibition of RNA translation or up-regulation of RNA degradation [Hathaway 2018].
Proposed mechanisms for mitochondrial disfunction involved in DbCM are summarised in the figure below (from Gollmer J, Diabetes Metab J 2020, Mitochondrial Mechanisms in Diabetic Cardiomyopathy)
These observations have led to an increased consideration of mitochondria as targets of novel therapies for DbCM. Some antidiabetic drugs, already validated in clinical practice, improve , directly or indirectly, mitochondrial dysfunction. Metformin, for instance, has been demonstrated to mitigate all mitochondrial abnormalities, at least by mitigating ER stress, a typical trait of DbCM [Chen 2017].
Recent discussions on this topic have heavily mentioned Sodium glucose cotransporter 2 inhibitors (SGLT2i). These inhibitors improve cardiovascular outcomes in diabetic subjects, with clinical studies showing dapaglifozin as beneficial for macrovascular outcomes, irrespective of glucose lowering. SGLT2i treatment may target myocardial mechanisms underlying systolic HF, some of which are also present in patients with DbCM, including mitochondrial defects. For instance, experimental studies revealed the cardiac Na+/H+-exchanger 1 as a target of SGLT2i, thus reducing cytosolic Na+ and Ca2+, increasing mitochondrial Ca2+ levels and thereby attenuating mitochondrial Ca2+ handling defects and re-establishing ATP regeneration [Uthman 2018].
Similar to SGLT2i, some glucagon-like peptide 1 receptor agonists (GLP1RA) have demonstrated a reduction of cardiovascular endpoints in clinical trials, thus resulting in a similar guideline recommendation as for SGLT2i [Marso 2016 a,b]. The underlying mechanism of GLP1RA cardiovascular effects were observed in a rat model, where GLP1RA diminish activation of mitochondrial apoptosis by preventing energy depletion, through reduction of oxidative stress [Qiao 2018].
As oxidative stress is a main cause of DbCM, mitochondrial ROS scavenging is an obvious potential treatment strategy. Several mitochondria-targeted agents have demonstrated the capacity to attenuate mitochondrial oxidative stress in preclinical studies, such as MitoQ, MitoTempol, or MnTBAP, showing antioxidant and anti-inflammatory effects [Escribano-Lopez 2016].
Some beneficial effects in DbCM may also be achieved by administering agents that modulate the myocardial (mitochondrial) substrate oxidation pattern towards a physiological substrate utilization pattern – e.g. reducing FAs oxidation - such as trimetazidine and ranolazine [Levelt 2018].
Finally, in a recently study, chronic oral application of the NAD+ precursor (nicotinamide adenine dinucleotide, oxidizing agent essential in the electron transfer reaction in all cells) nicotinamide riboside is able to elevate NAD+ levels in healthy adults, thereby opening a new treatment option for several cardiac diseases associated with decreased NAD+/NADH ratios, including DbCM [Martens 2018].
Improvement of myocardial mitochondrial abnormalities has been demonstrated through the use of cardioprotective antidiabetic drugs (e.g., SGLT2i, metformin) that appear to directly, or indirectly, mitigate the effects of defective mitochondria, thus ameliorating cardiovascular complications related to DbCM. This increased understanding of mitochondrial biology, in addition to the rapid development of cutting-edge biotechnologies, propels the hope that novel therapeutic options for DbCM, based on mitochondrial medicine, will be available in the near future.
Any Questions?
Latest activities
Latest news
Migraines: a point on mechanism-based and disease-specific therapeutics
Migraines are a common disabling headache disorder.Reproductive Medicine in the pandemic: the burden of COVID-19 on fertility treatments
The field of Reproductive Medicine saw many plans put on hold in the wake of Covid-19.About
Med-Ex Learning creates tailored programmes that provide the best Continuing Professional Development training and solutions for Healthcare industries and Healthcare professionals, everywhere.
Get in touch
Whether you have any queries about our activities, our approach or anything else, our team is happy to answer all your questions.